Many chronic illnesses lie hidden beneath the skin’s surface, making interrogation of disease initiation and progression a challenge.
To tackle this problem, a team of engineers at the University of California San Diego (UCSD) has developed a wearable sensor capable of detecting and continuously monitoring blood flow in deep tissues for cardiovascular diagnostics.
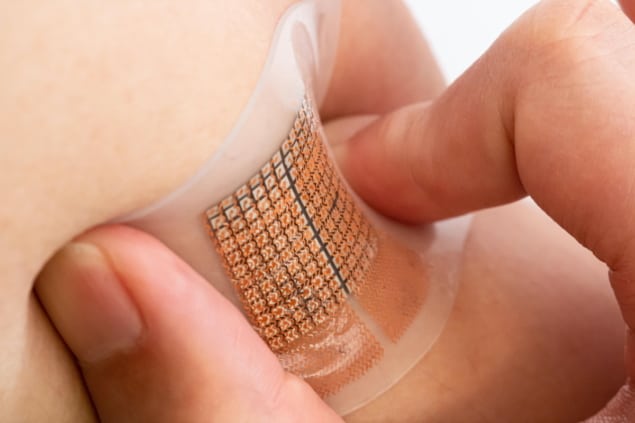
Wearable patches have seen much success when monitoring skin physiology at relatively shallow penetration depths. However, targeting deep tissues and resolving tissue-specific signals has previously posed an obstacle.
Such physiological signals arise from objects as small as a few micrometres, buried underneath strongly attenuating tissue layers. Therefore, a successful sensor must have both a deep probing depth and high spatial resolution.
Non-invasive sensor for deep tissue monitoring
The new patch, reported in Nature Biomedical Engineering, is capable of sensing microscale structures, such as red blood cells, from deep within the body and in real time. The valuable data obtained from the sensor may then aid clinicians in identifying cardiovascular problems at an early stage.
“This type of wearable device can give a more comprehensive, more accurate picture of what’s going on in deep tissues and critical organs like the heart and the brain,” explains principal investigator Sheng Xu, a professor of nanoengineering at the UCSD Jacobs School of Engineering.

How does it work?
The patch consists of a 12 x 12 array of millimetre-sized ultrasound transducers embedded in a thin stretchable polymer sheet. Importantly, it can sense and measure cardiovascular signals as deep as 14 cm inside the body. And by using an array of transducers, rather than a single transducer, the sensor can maintain a high degree of accuracy at this large penetration depth.
The sensors’ penetrative capabilities arise in part from controlling each transducer individually, known as phased-array technology. The transducers can either be operated synchronously, emitting a focused, high-intensity ultrasound beam, or asynchronously, which allows the beams to be steered to different angles.
While conventional wearable devices need to be moved and replaced to sense different regions, the beams of the phased array can be actively steered between a tilting angle of –20° to 20°. By propagating the ultrasonic waves at different angles, a larger region can be monitored than just the tissue directly beneath the patch.
“With the phased-array technology, we can manipulate the ultrasound beam in the way that we want,” says co-first author Muyang Lin, a nanoengineering PhD student at UCSD. “This gives our device multiple capabilities: monitoring central organs, as well as blood flow, with high resolution. This would not be possible using just one transducer.”
Owing to its soft mechanical properties, the patch can be worn, on the neck or the chest, for prolonged periods of time with minimal obstruction to the motion of the user. This capacity for long term, continuous monitoring can produce valuable data to help clinicians diagnose various cardiovascular diseases, including heart valve dysfunction, poor circulation, and blockages and clots that may cause heart attacks.
Body-coupled energy harvesting can power multiple wearable devices
In tests, the patch performed on par with commercial ultrasound probes typically used in clinics. Unlike commercial probes, the wearable sensor eliminates the need for a trained technician for operation. This reduces the labour involved and the risk of human error, while also presenting a promising pathway for point-of-care and at-home diagnostics.
Data transfer and power supply for the preliminary prototype rely on wires connecting the front-end sensor to the back-end acquisition and processing system. Now that the researchers have proved the accuracy of their sensor, they plan to focus on developing wireless capabilities that will allow users to go about their daily activities while recording data.




